For HLA-B*5701-negative adult patients with HIV-1. See Full Indication.
Importance of HLA-B✶5701 Screening
TRIUMEQ is for your HLA-B✶5701–negative patients with HIV-1.
Help reduce the risk of hypersensitivity reaction (HSR) to abacavir (ABC) and personalize patient care.
Importance of HLA-B✶5701 screening
- Serious and sometimes fatal HSRs have been associated with ABC and ABC-containing products
- The presence of the HLA-B✶5701 allele has been associated with a higher risk for HSR
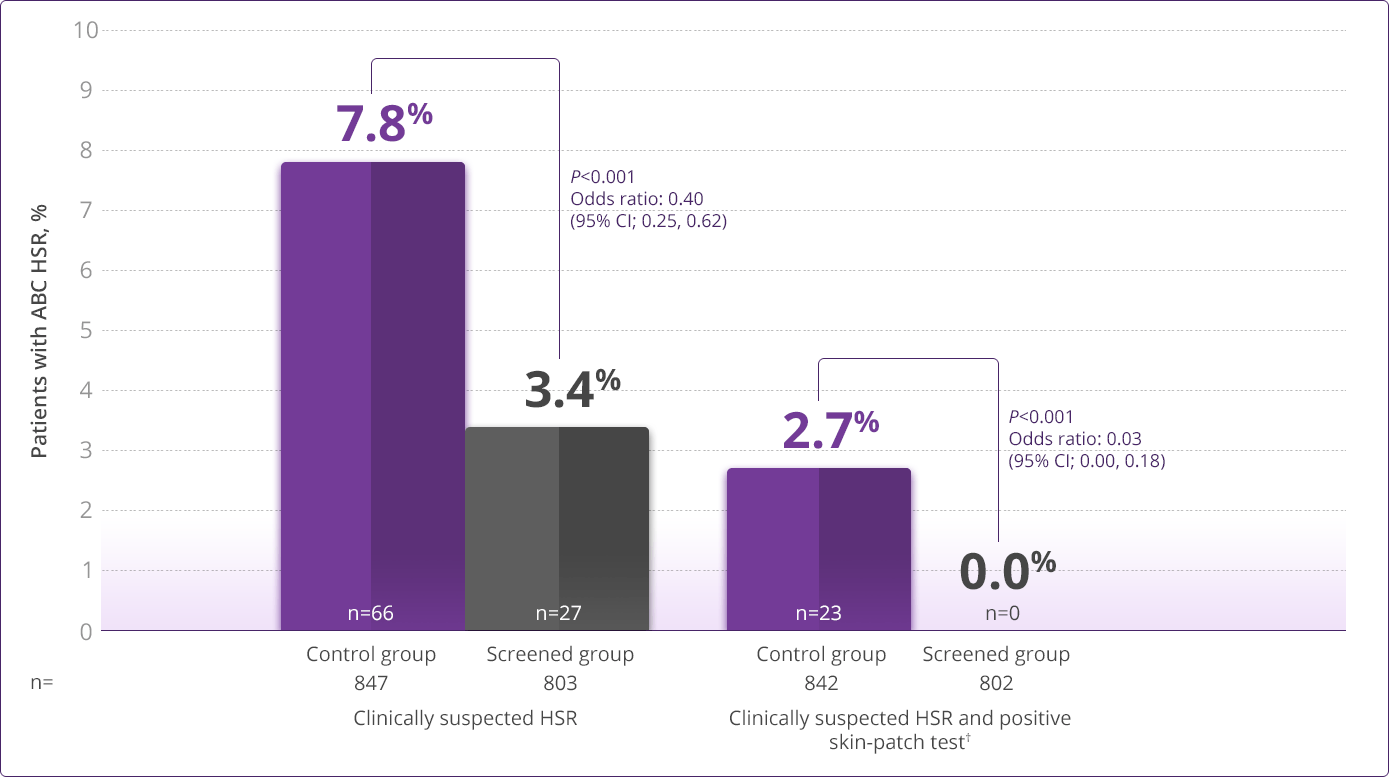
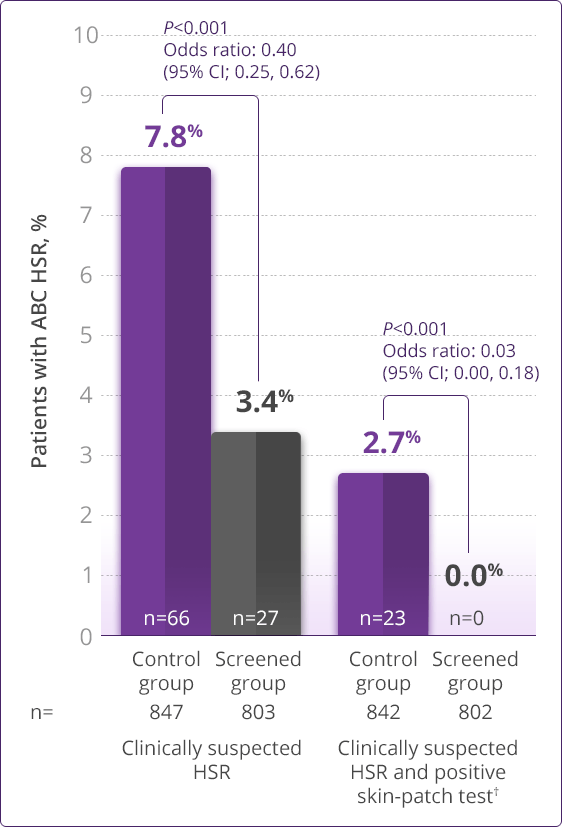
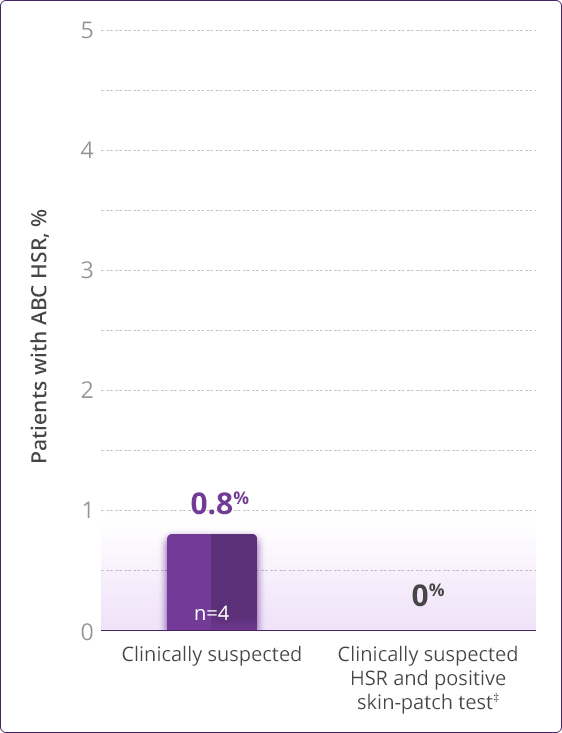
- HLA-B✶5701 screening significantly reduces the risk of an HSR to ABC1
- HLA-B✶5701 screening should not be used as a substitute for clinical judgment or pharmacovigilance, because a negative HLA-B✶5701 result does not eliminate the possibility of an ABC HSR2
- Screening is recommended by DHHS guidelines2
- DHHS guidelines recommend screening ABC-naïve patients for HLA-B✶5701 status before initiating an ABC-based regimen to reduce the risk of an HSR
- TRIUMEQ is contraindicated in HLA-B✶5701–positive patients
- Test result should be recorded and maintained, so it is only needed once in a patient's lifetime2
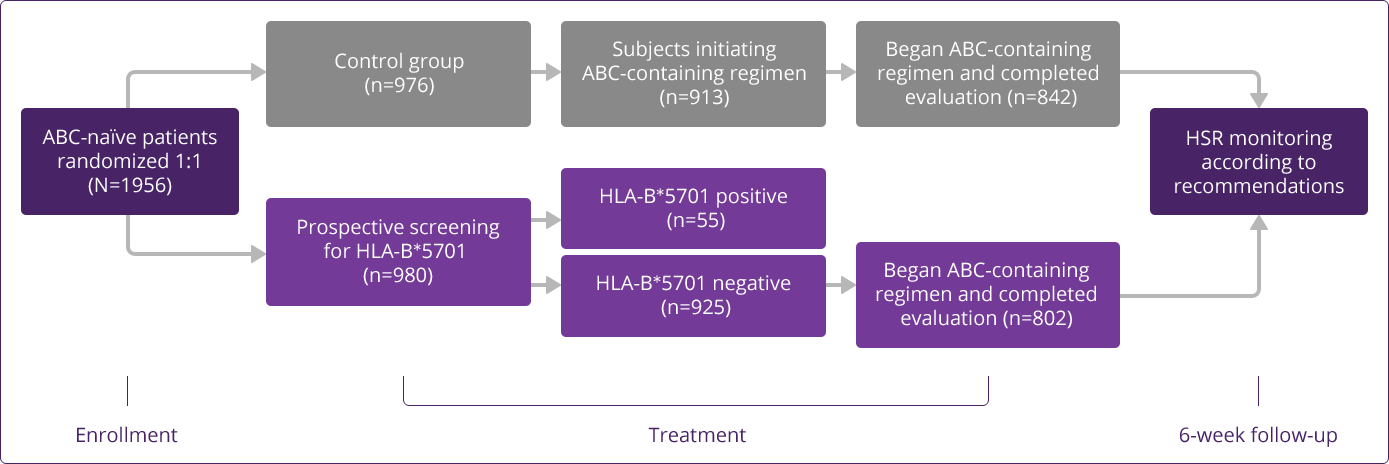
Prospective HLA-B✶5701 screening is the first step when considering an ABC-containing regimen.
HLA-B✶5701–negative patients may develop an HSR to ABC; however, this occurs significantly less frequently than in HLA-B✶5701–positive patients.
Overview of HLA-B✶5701 screening
- One-time test—HLA-B✶5701 screening is a genetic test, so a patient only needs to be tested once when results are documented in their medical record
- Helps provide prescribing assurance—Screening for the HLA-B✶5701 allele helps you determine which patients may be appropriate candidates for an ABC-containing regimen
- Screening guidelines—DHHS guidelines recommend screening for the HLA-B✶5701 allele before starting any patient on an ABC-containing regimen to decrease the risk of an HSR2
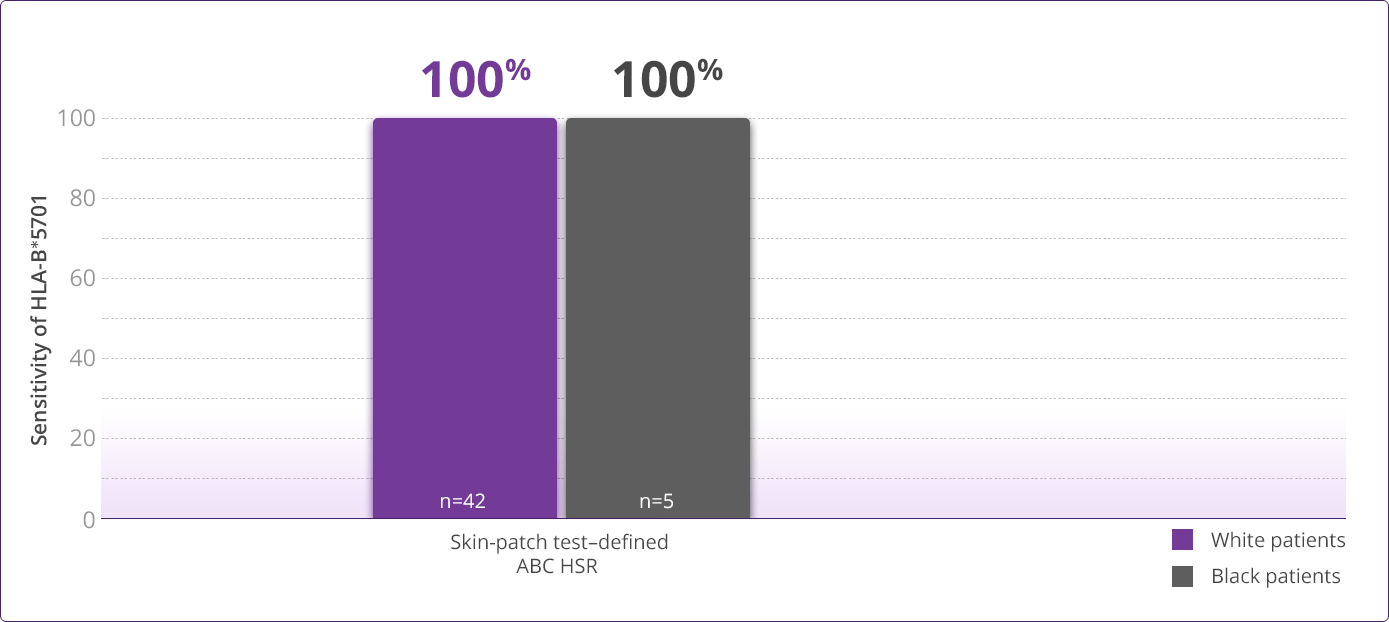
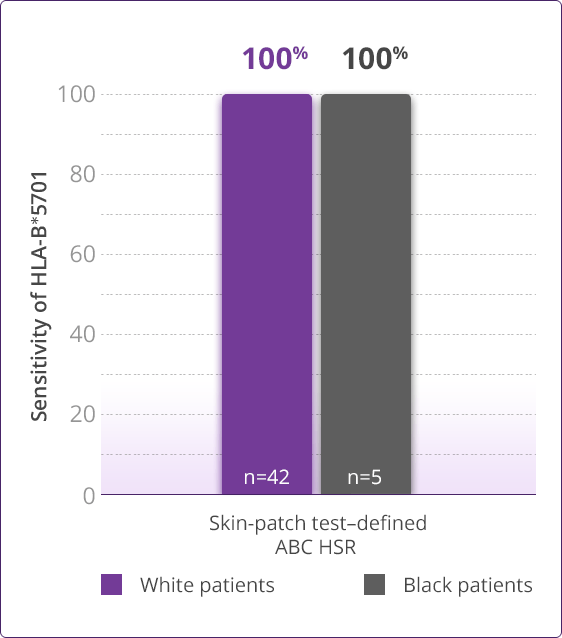
- Clinically demonstrated—Multiple clinical trials have established the value, utility, and accuracy of screening for the HLA-B✶5701 allele1,3-6
Always be sure to:
- Screen all patients for the HLA-B✶5701 allele prior to initiating therapy with ABC or reinitiation of ABC therapy in patients who discontinued ABC for reasons other than a suspected HSR, unless patients have a previously documented HLA-B✶5701 allele assessment
- Record HLA-B✶5701 status in patients' medical records
- Maintain clinical vigilance for ABC HSR, regardless of HLA-B✶5701 test results, because a negative result does not eliminate the possibility of an HSR2
- Note that when therapy with TRIUMEQ has been discontinued for reasons other than symptoms of an HSR, and if reinitiation of TRIUMEQ or any other ABC-containing product is under consideration, carefully evaluate the reason for discontinuation of TRIUMEQ to ensure that the patient did not have symptoms of an HSR
- Permanently discontinue ABC if HSR cannot be ruled out even when other diagnoses are possible, regardless of HLA-B✶5701 status
Do not perform HLA-B✶5701 testing to:
- Diagnose an ABC HSR
- Support a decision to rechallenge with ABC after a suspected HSR
- Following an HSR to ABC, NEVER restart TRIUMEQ or any other ABC-containing product because more severe symptoms can occur within hours and may include life-threatening hypotension and death

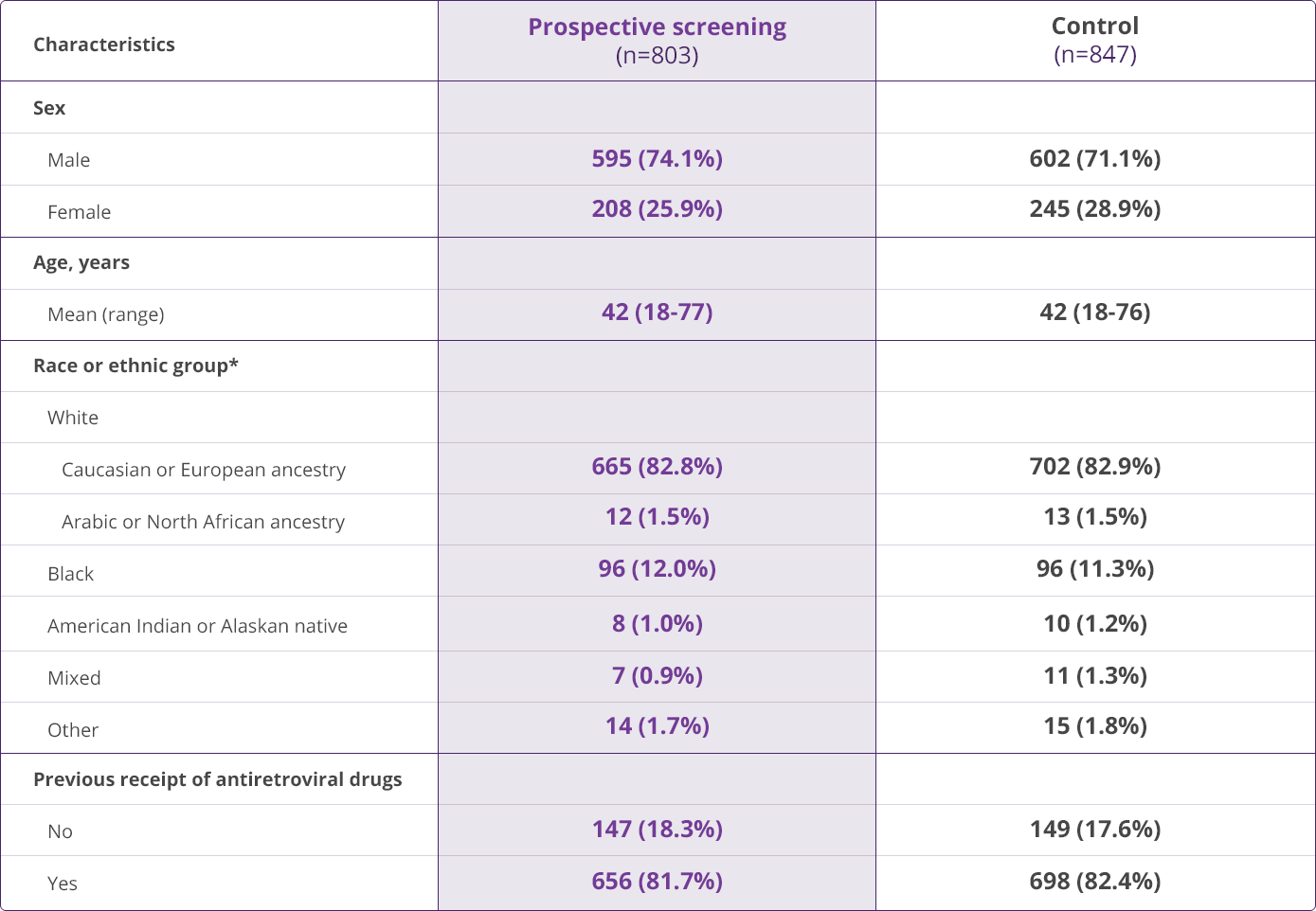
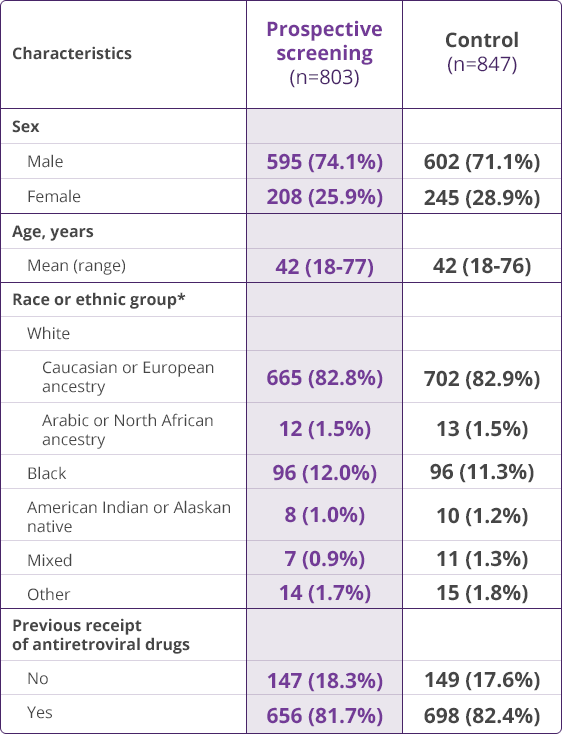
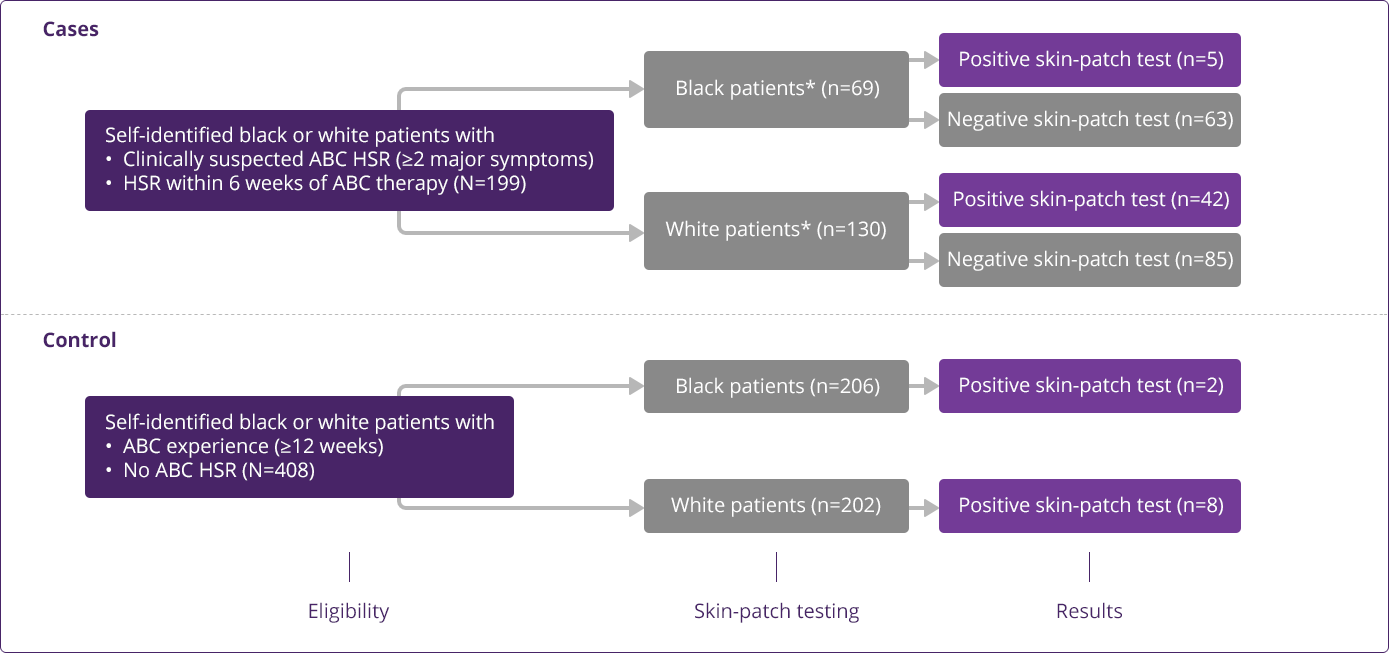
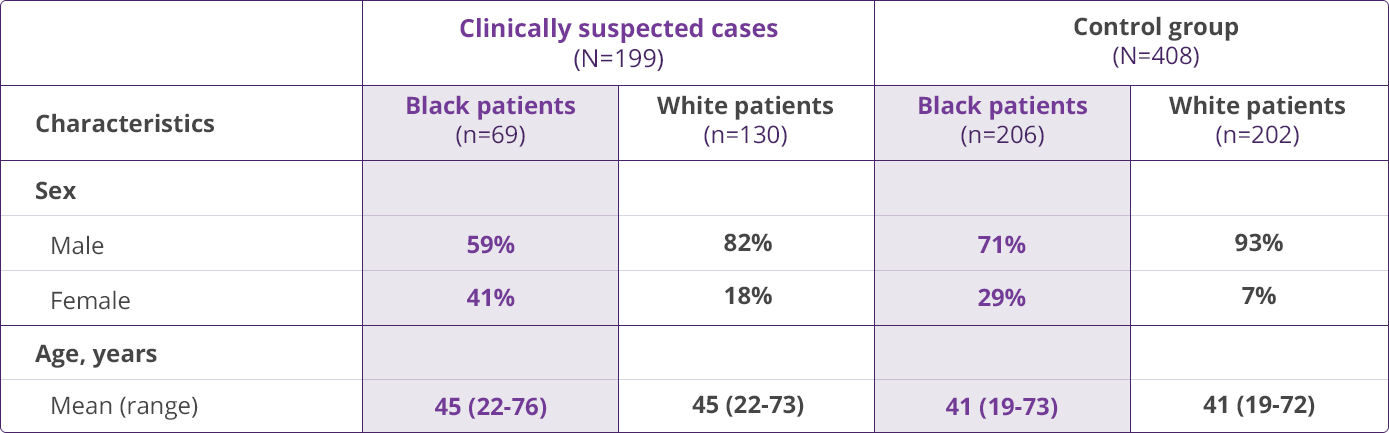
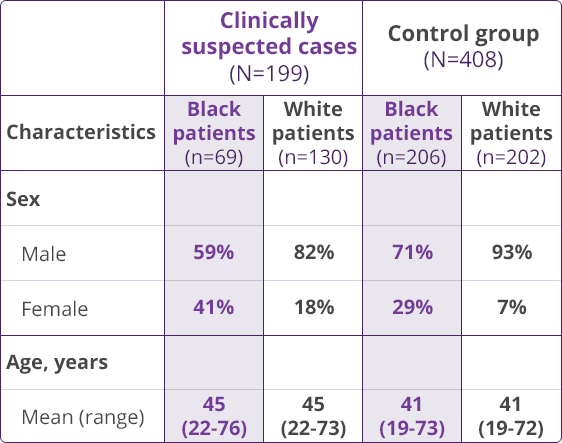
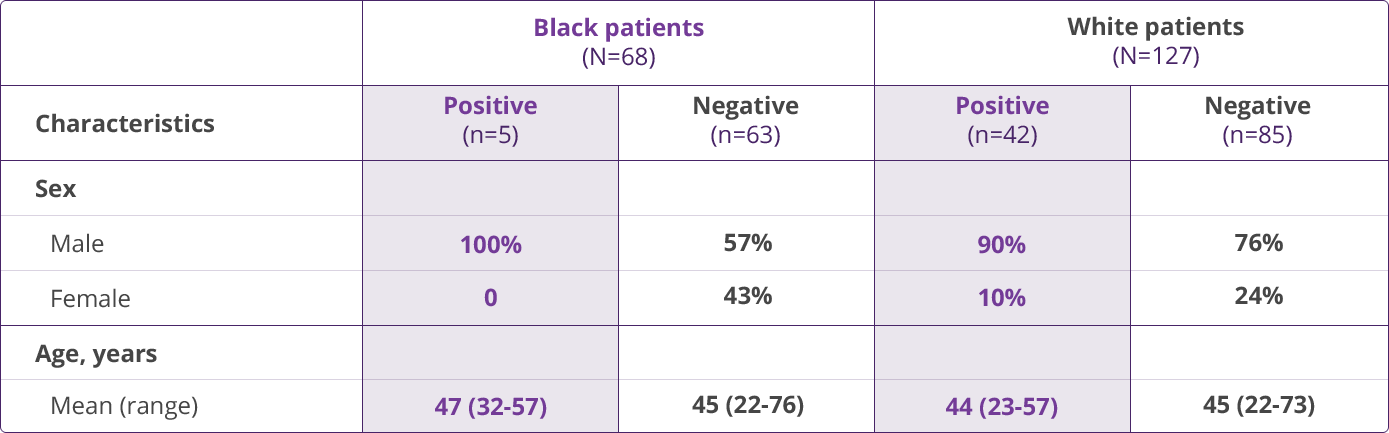
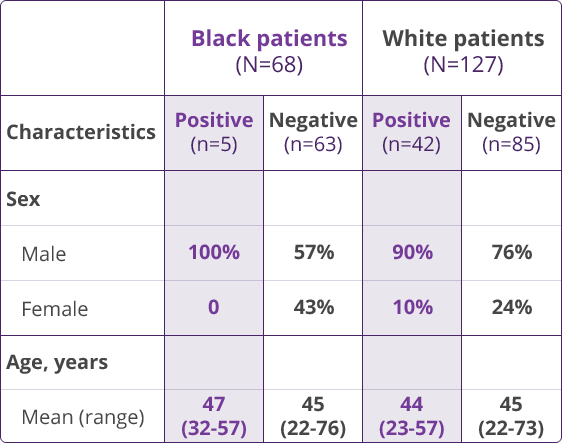
HLA-B✶5701 Clinical Studies


The Warning Card for TRIUMEQ
Patients should be screened for HLA-B*5701 before starting TRIUMEQ or any ABC-containing product
- It is important to know the result of a patient's HLA‐B✶5701 screening and enter the result in the patient's medical record
- HLA-B✶5701–positive patients should not take TRIUMEQ or any ABC-containing product
- Patients who test negative for HLA-B✶5701 can still develop an HSR
Patients will receive the Warning Card and Medication Guide at the pharmacy each time they pick up their prescription for TRIUMEQ. Instruct the patient to read the Medication Guide and Warning Card every time to obtain any new information that may be present about TRIUMEQ. The symptoms of ABC HSR are listed on the Warning Card for patient reference. Instruct patients to carry this Warning Card with them at all times.
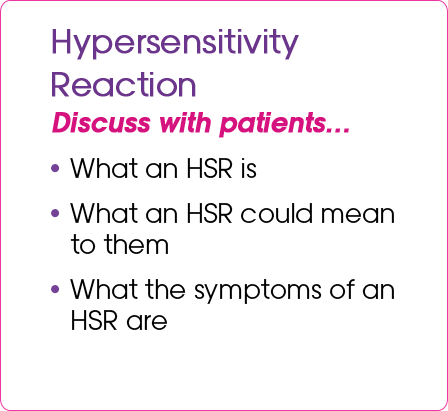
An important patient conversation
- Have a conversation with your patients with HIV-1 about their HLA-B✶5701 screening test results and the Warning Card, a valuable resource in HIV-1 treatment management
- It's important that patients understand the information the Warning Card contains and recognize the importance of carrying it with them at all times
The following are important points to cover with appropriate patients with HIV-1 about the HLA-B✶5701 test and the Warning Card


Please see full Prescribing Information, including Boxed Warning and Medication Guide, for TRIUMEQ and TRIUMEQ PD.
References
- Mallal S, Phillips E, Carosi G, et al; PREDICT-1 Study Team. HLA‐B✶5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568-579.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Updated March 23, 2023. Accessed August 24, 2023. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf
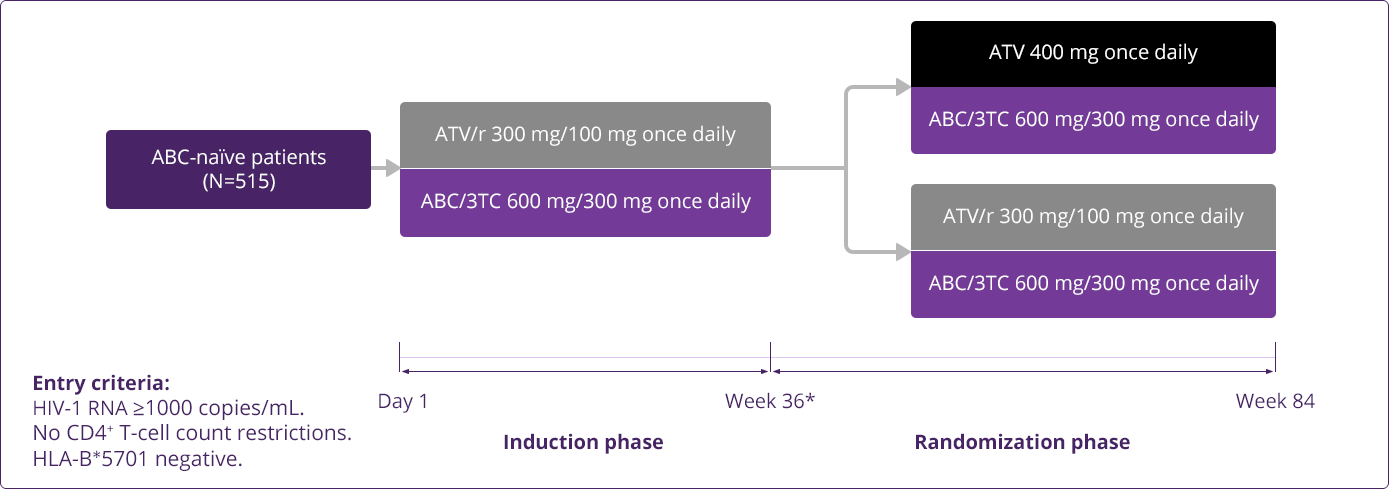
- Squires KE, Young B, DeJesus E, et al; for the ARIES Study Team. Safety and efficacy of a 36-week induction regimen of abacavir/lamivudine and ritonavir-boosted atazanavir in HIV-infected patients. HIV Clin Trials. 2010;11(2):69-79.
- Young B, Squires K, Patel P, et al. First large, multicenter, open-label study utilizing HLA‐B✶5701 screening for abacavir hypersensitivity in North America. AIDS. 2008;22(13):1673-1675.
- Small CB, Wohl D, Margolis DA, et al. Prevalence of HLA‐B✶5701 allele in HIV-infected subjects in North America and reductions in risk for development of abacavir associated hypersensitivity reaction. Presented at: 52nd International Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 9-12, 2012; San Francisco, CA. Poster H-895.
- Saag M, Balu R, Phillips E, et al. High sensitivity of human leukocyte antigen-B✶5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111-1118.
- Squires KE, Young B, DeJesus E, et al; for the ARIES Study Team. Similar efficacy and tolerability of atazanavir compared with atazanavir/ritonavir, each with abacavir/lamivudine after initial suppression with abacavir/lamivudine plus ritonavir-boosted atazanavir in HIV-infected patients. AIDS. 2010;24(13):2019-2027.
- Data on file, ViiV Healthcare.
DALWCNT230003