INDICATION
TRIUMEQ and TRIUMEQ PD are a combination of dolutegravir (integrase strand transfer inhibitor [INSTI]), abacavir, and lamivudine (both nucleoside analogue reverse transcriptase inhibitors) indicated for the treatment of HIV-1 infection in adults and in pediatric patients aged at least 3 months and weighing at least 6 kg.
Limitations of Use:
TRIUMEQ and TRIUMEQ PD alone are not recommended in patients with resistance-associated integrase substitutions or clinically suspected INSTI resistance because the dose of dolutegravir in TRIUMEQ and TRIUMEQ PD is insufficient in these subpopulations. See the dolutegravir prescribing information.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: HYPERSENSITIVITY REACTIONS AND EXACERBATIONS OF HEPATITIS B VIRUS (HBV)
Hypersensitivity Reactions:
- Serious and sometimes fatal hypersensitivity reactions, with multiple organ involvement, have occurred with abacavir-containing products
- Patients who carry the HLA-B✶5701 allele are at a higher risk of experiencing a hypersensitivity reaction to abacavir, although hypersensitivity reactions have occurred in patients who do not carry the HLA-B✶5701 allele
- TRIUMEQ and TRIUMEQ PD are contraindicated in patients with a prior hypersensitivity reaction to abacavir and in HLA-B✶5701-positive patients. All patients should be screened for the HLA-B✶5701 allele prior to initiating therapy or reinitiation of therapy with TRIUMEQ or TRIUMEQ PD unless patients have a previously documented HLA-B✶5701 allele assessment
- Discontinue TRIUMEQ or TRIUMEQ PD immediately if a hypersensitivity reaction is suspected, regardless of HLA-B✶5701 status and even when other diagnoses are possible
- Following a hypersensitivity reaction to TRIUMEQ or TRIUMEQ PD, NEVER restart TRIUMEQ or TRIUMEQ PD or any other abacavir-containing product
Exacerbations of Hepatitis B:
- All patients with HIV-1 should be tested for the presence of hepatitis B virus (HBV) prior to or when initiating TRIUMEQ or TRIUMEQ PD. Emergence of lamivudine-resistant HBV variants associated with lamivudine-containing antiretroviral regimens has been reported. If TRIUMEQ or TRIUMEQ PD is used in patients co-infected with HIV-1 and HBV, additional treatment should be considered for appropriate treatment of chronic HBV; otherwise, consider an alternative regimen
- Severe acute exacerbations of HBV have been reported in patients who are co-infected with HBV and HIV-1 and have discontinued lamivudine, a component of TRIUMEQ and TRIUMEQ PD. Monitor hepatic function closely in these patients and, if appropriate, initiate anti-hepatitis B treatment
Contraindications
- Do not use TRIUMEQ or TRIUMEQ PD in patients who have the HLA-B✶5701 allele
- Do not use TRIUMEQ or TRIUMEQ PD in patients with previous hypersensitivity reaction to abacavir, dolutegravir, or lamivudine
- Do not use TRIUMEQ or TRIUMEQ PD in patients receiving dofetilide
- Do not use TRIUMEQ or TRIUMEQ PD in patients with moderate or severe hepatic impairment
Warnings and precautions
Hypersensitivity Reactions:
- Hypersensitivity reactions have been reported with dolutegravir and were characterized by rash, constitutional findings, and sometimes organ dysfunction, including liver injury
- Clinically, it is not possible to determine whether a hypersensitivity reaction with TRIUMEQ or TRIUMEQ PD would be caused by abacavir or dolutegravir
- Discontinue TRIUMEQ or TRIUMEQ PD immediately if signs or symptoms of hypersensitivity reactions develop, as a delay in stopping treatment may result in a life-threatening reaction. Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated
Hepatotoxicity:
- Hepatic adverse events have been reported, including cases of hepatic toxicity (elevated serum liver biochemistries, hepatitis, and acute liver failure), in patients receiving a dolutegravir-containing regimen without pre-existing hepatic disease or other identifiable risk factors
- Patients with underlying hepatitis B or C may be at increased risk for worsening or development of transaminase elevations with use of TRIUMEQ or TRIUMEQ PD. In some cases, the elevations in transaminases were consistent with immune reconstitution syndrome or hepatitis B reactivation, particularly in the setting where anti-hepatitis therapy was withdrawn
- Drug-induced liver injury leading to liver transplant has been reported with TRIUMEQ
- Monitoring for hepatotoxicity is recommended
Lactic Acidosis and Severe Hepatomegaly With Steatosis:
- Fatal cases have been reported with the use of nucleoside analogues, including abacavir and lamivudine. Discontinue TRIUMEQ or TRIUMEQ PD if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity develop, including hepatomegaly and steatosis in the absence of marked transaminase elevations
Embryo-Fetal Toxicity:
- Assess the risks and benefits of TRIUMEQ and discuss with the patient to determine if alternative treatments should be considered at the time of conception through the first trimester of pregnancy due to the risk of neural tube defects
- Pregnancy testing is recommended before use of TRIUMEQ. Adolescents and adults of childbearing potential should be counseled on the consistent use of effective contraception
- TRIUMEQ may be considered during the second and third trimesters of pregnancy if the expected benefit justifies the potential risk to the pregnant woman and the fetus
Adverse Reactions or Loss of Virologic Response Due to Drug Interactions with concomitant use of TRIUMEQ or TRIUMEQ PD and other drugs may occur (see Contraindications and Drug Interactions).
Immune Reconstitution Syndrome, including the occurrence of autoimmune disorders with variable time to onset, has been reported with the use of TRIUMEQ or TRIUMEQ PD.
Different Formulations Are Not Interchangeable:
TRIUMEQ and TRIUMEQ PD are not bioequivalent and are not interchangeable on a milligram-per-milligram basis. If a patient switches from one formulation to the other, the dose must be adjusted.
Myocardial Infarction (MI):
- Several observational studies have reported an association with the use of abacavir and the risk of MI; meta-analyses of randomized controlled clinical trials did not show increased risk. To date, there is no established biological mechanism to explain a potential increase in risk. In totality, the available data show inconsistency; therefore, evidence for a causal relationship between abacavir and the risk of MI is inconclusive
- The underlying risk of coronary heart disease should be considered when prescribing antiretroviral therapies, including abacavir, and action taken to minimize all modifiable risk factors (e.g., hypertension, hyperlipidemia, diabetes mellitus, smoking)
Adverse reactions
- The most common adverse reactions (incidence ≥2%, Grades 2-4) in treatment-naïve adults receiving TRIUMEQ were insomnia (3%), headache (2%), and fatigue (2%)
Drug interactions
- Coadministration of TRIUMEQ or TRIUMEQ PD with other drugs can alter the concentration of other drugs and other drugs may alter the concentrations of TRIUMEQ or TRIUMEQ PD. The potential drug-drug interactions must be considered prior to and during therapy
- Drugs that induce or inhibit CYP3A or UGT1A1 may affect plasma concentrations of dolutegravir
- Administer TRIUMEQ 2 hours before or 6 hours after taking antacids, polyvalent cation-containing products or laxatives, sucralfate, oral supplements containing iron or calcium, or buffered medications. Alternatively, TRIUMEQ and supplements containing calcium or iron can be taken with food
Use in specific populations
- Pregnancy: Assess the risks and benefits of TRIUMEQ and discuss with the patient to determine if an alternative treatment should be considered at the time of conception through the first trimester or if pregnancy is confirmed in the first trimester due to the risk of neural tube defects
- Lactation: Breastfeeding is not recommended due to the potential for HIV-1 transmission, developing viral resistance in HIV-positive infants, and adverse reactions in a breastfed infant
- Females and Males of Reproductive Potential: Pregnancy testing is recommended before initiation of TRIUMEQ. Counsel adolescents and adults of childbearing potential taking TRIUMEQ on the consistent use of effective contraception
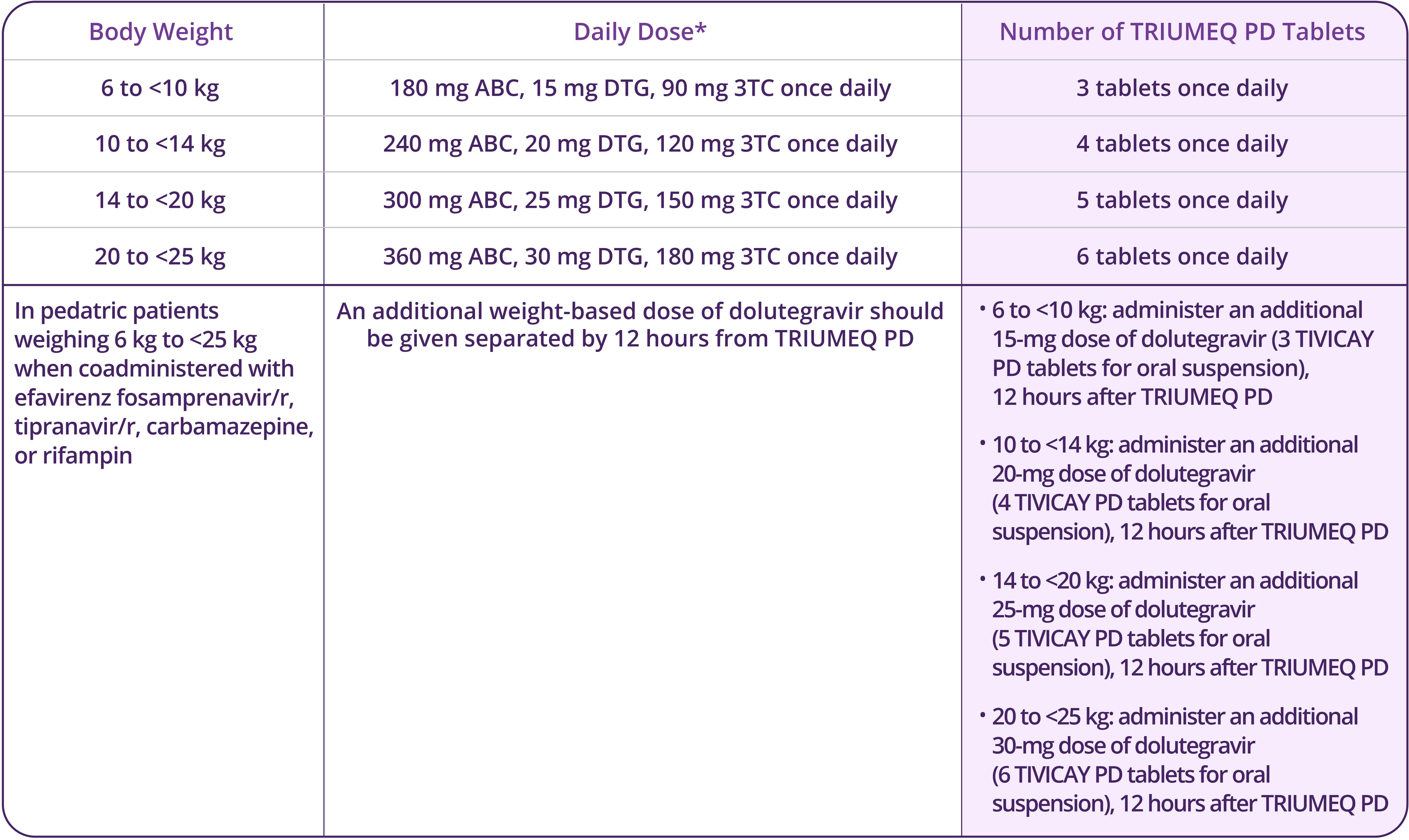
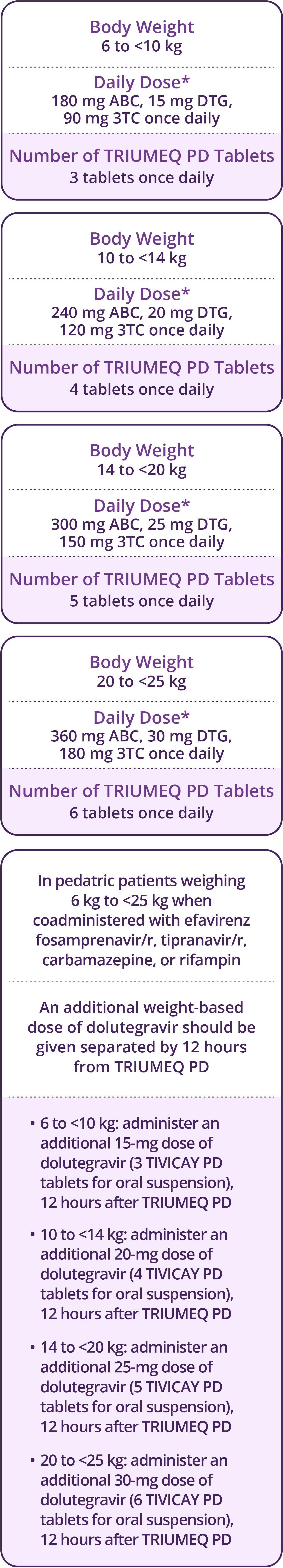
- Pediatrics: Not recommended for patients aged <3 months or weighing <6 kg
- Impaired Renal Function: TRIUMEQ and TRIUMEQ PD are not recommended for patients with creatinine clearance <30 mL/min and pediatric patients with a similar degree of renal impairment based on age-appropriate assessment of renal function. Patients with a sustained creatinine clearance between 30 and 49 mL/min should be monitored for hematologic toxicities, which may require a dosage adjustment of lamivudine as an individual component
- Impaired Hepatic Function: If a dose reduction of abacavir is required for patients with mild hepatic impairment, then the individual components of TRIUMEQ or TRIUMEQ PD should be used
INDICATION
TRIUMEQ and TRIUMEQ PD are a combination of dolutegravir (integrase strand transfer inhibitor [INSTI]), abacavir, and lamivudine (both nucleoside analogue reverse transcriptase inhibitors) indicated for the treatment of HIV-1 infection in adults and in pediatric patients aged at least 3 months and weighing at least 6 kg.
Limitations of Use:
TRIUMEQ and TRIUMEQ PD alone are not recommended in patients with resistance-associated integrase substitutions or clinically suspected INSTI resistance because the dose of dolutegravir in TRIUMEQ and TRIUMEQ PD is insufficient in these subpopulations. See the dolutegravir prescribing information.
Please see full Prescribing Information, including Boxed Warning and Medication Guide, for TRIUMEQ and TRIUMEQ PD.
DALWCNT230002